
Microphysiological systems: analysis of the current status, challenges and commercial future
Introduction
Microphysiological systems (also termed organs-on-a-chip) hold the promise to improve and expedite the overall drug discovery pipeline. The rise of the field has been due to several factors including the continuous rising costs of developing new pharmaceuticals, the pressure to reduce animal experimentation, and the need for more predictive and high-throughput in vitro models. In the past decade, the advancement of microfluidics technologies has facilitated the development of microphysiological systems as simple, reproducible, and scalable platforms that recapitulate organ-level functions. The miniaturized biomimetic organ models, when interconnected together in a microfluidic circuit, can further recapitulate the physiology, compartmentalization, and interconnectivity of the human system. This then potentially enables the accurate prediction of human responses towards pharmaceutical compounds, and the development and testing of nanomedicines, chemicals, and biological species (1-3).
Several initiatives and companies are operating to increase the adoption of these technologies more broadly by industry. In this review we summarize the key developments in the field of microphysiological systems, debate the challenges towards adoption by stakeholders, and analyze available data regarding the commercialization of such systems.
Methods
The companies included in this study are listed in Table 1 and were obtained from web searches until June 20th 2018 including “organ-chip” keywords, as well as from other publications (4-6). The study is limited to the set of companies listed here, hence conclusions take into account only this information. In addition, funding or partnership information are sometimes unavailable to the public and it is one of the limitations of this review. Company information such as founding year, location, and target organ models were obtained from their respective websites. Company size was determined by the number of full-time employees (FTEs) based on their LinkedIn company profile (ranges 1–10, 11–50, and 51–100). Funding and partnership information was obtained from press releases on company websites as well as Crunchbase and Pitchbook databases (www.crunchbase.com, www.pitchbook.com). Companies active in the area of organoids, 3D bioprinting, or in the marketing of microfluidics systems that do not include cellular components, were not included in this analysis. The number of articles per year for different topics (“organ-on-a-chip”, “microfabrication”, “lab-on-a-chip”, and “CRISPR”) were obtained from Web of Science (www.isiwebofknowledge.com
Table 1
| Company | Founding year | Location | Organ/model | Partnerships | Website |
|---|---|---|---|---|---|
| 4Design Biosciences | 2014 | Corona del Mar, CA, USA | Cancer cell lines, cardiomyocytes, neural stem cells, hematopoietic stem cells, vasculature | – | www.4designbiosciences.com |
| AIM Biotech | 2012 | Singapore, Singapore | Stem cells, cancer, vasculature, neural cells | Distribution: Fisher Scientific Pte Ltd, Flexcell® International Corporation, Tebu-bio, Funakoshi Co., Ltd., Hangzhou Jiuyang Biotechnology Co., Ltd. (JoyingBio), Woongbee MeDiTech Inc. | www.aimbiotech.com |
| AlveoliX AG | 2015 | Bern, Switzerland | Lung-on-Chip | – | www.alveolix.com |
| Ascendance Biotechnology, Inc. (formerly known as Hepregen Corporation) | 2007 | Medford, MA, USA | Liver | – | www.ascendancebio.com |
| AxoSim | 2014 | New Orleans, LA, USA | Nerve-on-Chip | – | www.axosim.com |
| BI/OND Solutions B.V. | 2017 | Delft, The Netherlands | Cell lines, cardiomyocytes | – | www.gobiond.com |
| CN Bio Innovations | 2009 | Welwyn Garden City, UK | Single-Organ and Multi-Organ-Chip: 10-, 7-organ human body-on-a-chip model; a liver-on-a-chip model of cancer metastasis, a full viral lifecycle organ-chip model of hepatitis B and a liver-on-a-chip model of non-alcoholic fatty liver disease and steatohepatitis (NAFLD/NASH) | AstraZeneca, FDA’s Centre for Drug Evaluation and Research, Alnylam Pharmaceuticals, Isis Pharmaceuticals, Benitec Biopharma | www.cn-bio.com |
| EmulateBio | 2013 | Boston, MA, USA | Lung, intestine, liver, kidney, bone marrow | Roche AG, Takeda Pharmaceutical Company Limited, Cedars-Sinai, FDA, Janssen Research & Development LLC., Covance, Seres Therapeutics, MSD | www.emulatebio.com |
| HemoShear Therapeutics | 2008 | Charlottesville, VA, USA | Models of nonalcoholic steatohepatitis (NAFLD/NASH), propionic acidemia and methylmalonic acidemia | Takeda Pharmaceutical Company Limited, UVA Health System, Pfizer Inc., Medivir AB | www.hemoshear.com |
| Hesperos Inc. | 2015 | Orlando, FL, USA | Multi-Organ-Chips (skin, endocrine, lung, intestine, lung, brain, heart, liver, bone marrow, kidney, pancreas), heart, muscle, liver, blood brain barrier | L’Oréal | www.hesperosinc.com |
| Hµrel Corp. | 2006 | North Brunswick, NJ, USA | Liver (hepatitis B disease model, hepatic co-cultures, hepatotoxicity screening, various primary hepatic co-cultures within one model, continuous monitoring) | Bristol-Myers Squibb, ACEA Bioscience, Astra Zeneca, Optivia Biotechnology, Quintiles, Sanofi US, UCB | www.hurelcorp.com |
| Kirkstall Ltd | 2006 | Rotherham, UK | Liver, kidney, intestine, blood-brain barrier, heart, lung | Distribution agreement with Lonza | www.kirkstall.com |
| Kiyatec | 2005 | Greenville, SC, USA | Breast cancer, glioblastoma, lung, ovarian, pancreatic cancer | Greenville Health System’s Institute for Translational Oncology Research, Distribution: Sigma-Aldrich | www.kiyatec.com |
| Mimetas | 2013 | Leiden, The Netherlands | Breast cancer, neuronal networks, intestine, liver, blood brain barrier, kidney, vasculature, glioma, placenta models | Molecular Devices, Roche, Pfizer and GlaxoSmithKline, Galapagos, Biogen, Abbvie and Janssen | www.mimetas.com |
| Nortis Bio | 2012 | Seattle, WA, USA | Blood-brain barrier, vascular injury, kidney, liver, heart, intestine, cancer, and stem cell models | – | www.nortisbio.com |
| Quorum Technologies Inc. | 1987 | Puslinch, ON, USA | Vasculature | – | www.quorumtechnologies.com |
| SynVivo | 2014 | Huntsville, AL, USA | Cancer model, blood-brain barrier model, inflammation model, toxicology model | Distribution: Cosmo Bio Co. Ltd., Nakayama Co. Ltd., JSK Biomed, MolDiag Solutions, Lavi Med-Tech, Stratech Scientific Ltd., LuBioScience, PELOBiotech, Tebu-Bio | www.synvivobio.com |
| Tara Biosystems | 2014 | New York, NY, |
Heart-on-Chip | - | www.tarabiosystems.com |
| TissUse | 2010 | Berlin, Germany | Adipose tissue, vasculature, bone marrow, liver, kidney, pancreas, intestine, brain, lung, heart, hair follicle, lymph node, skin | Roche, Astra Zeneca | www.tissuse.com |
Current status of organs-on-a-chip
The scientific output of organs-on-a-chip drastically increased in the past years, with an initial focus on recapitulating normal organ functions. For instance, different microfabrication techniques have allowed the creation of dynamic mechanical microenvironments to apply shear stress, strain, and/or interfaces on different cell types (1,3,7,8). The creation of a lung-on-a-chip platform boosted the field by integrating mechanical strain and interfacing multiple cell types mimicking the respiration of the lung (9,10). The mechanical strain simulated the cyclic motions experienced by the lung alveoli and revealed unique responses under strain not observed in static conditions. Other approaches have been pursued to simulate dynamic mechanical environments of the gut (11,12), heart (13-16), and blood vessels (8,17-20), among others.
More recently, there has been an increased emphasis on disease model generation and multi-organ systems (Figure 1). These approaches yielded new insights into biological processes not achievable with other technologies or animal models. For instance, several organ-on-a-chip approaches focused on developing disease models or states, such as Hutchinson-Gilford Progeria syndrome and hypertension (8), Barth syndrome (16), pulmonary edema (9), and atherosclerosis (19). These systems use human cells and are becoming an invaluable tool for drug discovery and toxicity screening; they are expected to lead to the development of new therapeutic agents. For instance, a progeria-on-a-chip model (8) was able to recapitulate an inflammatory state observed in patients, which was then reversed by using therapeutic agents in promising clinical trials (24). Besides contributing to mechanical control, microfluidics technologies proved useful in modeling several human barriers. Platforms have been created to mimic the blood-brain barrier (25-27), placental barrier (28) and blood-lymphatic vessel barrier (29). The development of these models is instrumental in the studies of drug and toxin transport across barriers as they provide simple yet robust systems to gain mechanistic insights into barrier disturbances. These are a few examples that illustrate the potential of using organ-chips during drug discovery.
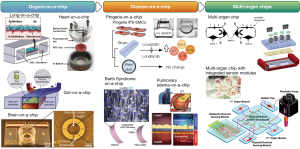
Enabling technologies are playing an important role in furthering of the organs-on-a-chip field. Lee and Cho recently used 3D printing to create a single-step-fabrication procedure that generates microfluidic circuits embedded with relevant cell types. Using this approach, they could create a liver-on-a-chip system by sequentially printing different materials, from cell-laden hydrogels to polycaprolactone (PCL)-based microfluidic channels (30). Another approach used 3D printing to directly print polydimethylsiloxane (PDMS) structures (31). The capabilities of 3D printing can be applied to generate fluidic channels for organs-on-a-chip but are also valuable in generating complex micro multi-tissue structures that can mimic several more complex organ functions (32). We envision these novel fabrication techniques to expedite the scalability of organs-on-a-chip. Although most organs-on-a-chip devices use PDMS as the base material, approaches relying on hydrogel microfluidics have emerged in the past few years [reviewed by Verhulsel et al. (33)]. Compared to the inert elastomers and plastics, hydrogels with different compositions allow for precise control over the extracellular matrix (ECM) properties, such as cell binding, stiffness, porosity, and degradability that directly tailor the cell behaviors in 3D microenvironments. The utilization of microfluidics based on hydrogels alone or in combination with PDMS (14) will potentiate the creation of biologically relevant organs-on-a-chip by providing additional microenvironmental cues.
The technical advantages of microfluidics also make it an ideal technology for the design and creation of multi-organ human-body-on-a-chip platforms. Here, the complexity of the fluidic architecture can mimic the required compartmentalization, nutrient gradient, and micro-scale distribution of organoids in a manner similar to those in vivo (34-36). Challenges still remain, as do opportunities to determine the rational design of a body-on-a-chip by precisely understanding the scale of each organ module and thus engineering of the microfluidics connecting these organoids (37,38). Early efforts towards a body-on-a-chip included systems with three to four organs or cell types that explored multiple organ interactions and metabolism (39-41). With the development in the last few years of novel and robust single organs-on-a-chip, we envision their combination into larger-scale multi-organ-on-a-chip systems featuring proper microfluidics routing and scaling (37,38). The different modules would have autocrine and paracrine communications, different organs would metabolize drugs or other agents to be tested, and multiple interactions could be identified (2). Since the replication at the level of a whole organism is challenging, these complex systems would allow studies of different diseases at a multi-organ level. Here, key organs known to be important players for a specific drug or compound would be represented, while using a simple and standardized setup. Complex microfluidic circuits would allow automation and integration of organ models with biosensors, which could evaluate organoid responses to various stimuli (23,38,42-44).
Challenges for adoption
Microfluidics research has come a long way in enabling the development of microphysiological systems. However, the progress and broad adoption of such systems still faces several challenges. Compatibility with existing equipment is crucial for academic and industrial research alike, where novel devices must operate with a minimum added equipment and work out-of-the-box with conventional microscopes, incubators, and other crucial equipment and techniques. Ease of handling is another factor influencing adoption, where end-users should be able to operate microphysiological systems without the need for extensive training or complex instructions. Here, design thinking approaches can be a tool to improve usability and downward end-user adoption. Several industries are moving towards automation, and microphysiological systems should be prepared to that future. This is especially crucial for high-throughput applications in pharmaceutical industry, where screenings for novel compounds are moving continuously towards automated systems. Some multi-organ systems have started to take automation in consideration by using computer-controlled arrays of microfluidic valves that manage fluid flow and sensor modules (23). Multiplexing is another challenge faced, but the inherent advantages of microfluidics make microphysiological systems a prime candidate for it. Organ- or multi-organ systems can be tested for multiple targets in real time, revealing dynamics and information not available up until now. With the rise of microphysiological systems we have seen a shift from normal organ modules to disease modules highlighting the importance of understanding biology and acquire the proper molecular and physiological readouts for applications in drug discovery. Most systems are still made out of PDMS, and there is a broader discussion in the field of what are the best materials to use. Where PDMS has traditionally been the material of choice due to its favorable properties with regard to fabrication and permeability, other materials like hydrogels may be more suitable in supporting cell growth. And when selecting the optimal material, other factors such as optical properties to allow for imaging and drug adsorption and absorption should also be taken into account. All of these challenges affect most stakeholders involved with microphysiological systems, but one additional factor receives a growing importance—validation. Regulatory agencies are closely monitoring the progress of the field and broader discussions are in place to understand how novel microphysiological systems should be validated and what would be the impact of that.
Commercial perspective and drivers (Figures 2,3)
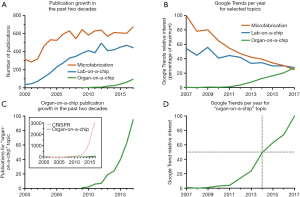
Since the field of organs-on-a-chip is relatively recent, most publications focus on new technologies and applications, with only a few dedicated to market adoption and commercialization efforts (4-6,45). Over the past decade we observed an increase in the number of publications containing the keywords “organ-on-a-chip” (Figure 2A). Although initially a very technology-oriented field, aiming to develop prototypes and concepts, the organ-on-a-chip sector is currently transitioning into a more commercial focus. Indeed, since 2012 the number of organ-on-a-chip companies on Table 1 more than doubled (Figure 3A). We hypothesize that since 2012, the field has grown and matured to a point of justifying venture investment for potential applications in drug discovery. At the same time, the global interest in the field increased. An analysis of the Google Trends topic “organs-on-a-chip” (Figure 2B,C,D) in web searches identified continuous growth, with a peak interest as of 2017 and mid-point in 2014 (Figure 2D). The Google Trend analysis also revealed that topics in a similar area (“microfabrication” and “lab-on-a-chip) have seen a steady decline reaching a similar level to “organs-on-a-chip”. Furthermore, the publications trend as seen for organs-on-a-chip differs from other developing technology fields like lab-on-a-chip, microfabrication or CRISPR. Analyses for these keywords show a more stable profile over the last decade for lab-on-a-chip and microfabrication (Figure 2A), indicating that the developments seen for organs-on-a-chip cannot be explained by a general rise of interest in new technologies. However, the total amount of publications and growth is significantly smaller compared to CRISPR (Figure 2C), which has attracted more research and overall funding.
A recent survey analyzed the opinions of stakeholders such as pharmaceutical companies and universities (4) regarding the use of organs-on-a-chip in drug discovery and development. Respondents to the survey predicted improvement possibilities in basic research, including target validation, while also considering pre-clinical development. Opinions diverged regarding clinical trials, with respondents from pharmaceutical companies mostly disagreeing on the replacement or reduction of human trials whereas university respondents where more divided (4). This study highlighted the relevance of understanding stakeholder opinions and needs early-on, to maximize the adoption and successful implementation of organ-on-a-chip technologies. Another important aspect relevant to the discussion is the business model behind such companies. Burgwal and co-workers analyzed different business models applicable to organs-on-a-chip companies and surveyed potential end-users (life science companies, pharmaceutical companies, contract research organizations (CROs), and universities/research institutes) (5). For most potential users, the preferred model included the product and a free basic startup service. An exception to this were pharmaceutical companies who weighed more relevance to models using CRO. Such information indicates that pharmaceutical companies might not be as willing to invest in new technologies for drug discovery, and instead prefer outsourcing it to CROs. Big pharmaceutical companies have been outsourcing innovation and research services to other companies, hence it is not surprising to observe the preference for CROs where they can rely on external expertise to obtain fast and trustworthy results. Despite differences in preferred business models, the majority of end-users agree on the relevance and future value of organ-on-a-chip technologies (5). However, the willingness to invest in new hardware is still limited, which can slow down adoption. In addition, most companies are operating with a hybrid business model that combines product and service offerings. Since the adoption is in early stages, end-users often require more specific solutions that need to be especially developed or tailored, which deviates organ-on-a-chip companies from a more scalable razor-blade model (5).
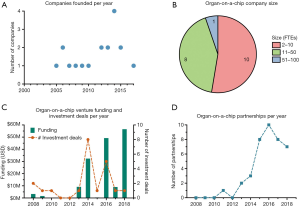
On Table 1 we list companies working more exclusively on organs-on-a-chip (not including 3D bioprinting or organoid companies). These companies are working on a variety of organ models and have already entered partnerships with bigger companies including well-known pharmaceutical companies. Most of the organ-on-a-chip companies are still young and relatively small in number of FTEs (Figure 3B). Venture funding and number of deals peaked in 2016 and 2018 (Figure 3C), and the results of such investments may still require more time to see fruition. We observed also an increase of funding per investment deal which indicates the maturation of the investments from seed stages to later stages (series A, B, and other types). Another indicator of the growth of the field are the increasing number of partnerships over the past decade (Figure 3D), where more and more pharmaceutical companies engage in partnership agreements geared towards drug discovery. It should be noted that information on partnership agreements and funding rounds is not always publicly available and therefore the data in Figure 3D and Table 1 cannot be seen as a complete overview of the adoption of organs-on-a-chip by the pharmaceutical industry. However, it is likely that an increase in the number of publicly communicated partnerships corresponds with an increase in the number of total partnerships. Overall, there has been a positive increase in venture funding and partnerships, highlighting a continuous investment in the area and commercial potential. At the same time several governmental grant schemes have been created to specifically support the field, such as the Tissue Chip Discovery Program from the National Institutes of Health.
Conclusions and future outlook
The organs-on-a-chip field has seen an increase in scientific output (publications) but also in the number of startup companies created and total funds raised. Early assessment of potential end-users and stakeholders point towards the importance of the technology in drug discovery and development, with a special emphasis on discovery and pre-clinical stages. The next 5–10 years will be crucial for the technology to overcome challenges with standardization and regulatory endorsement. Judging by the number of partnerships between established corporations and organ-on-a-chip companies we believe the field will help mitigate current business needs in the drug discovery arena and consequently achieve commercial success.
Acknowledgments
Funding: J Rouwkema received financial support from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (No. 724469).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/mps.2018.10.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol 2014;32:760-72. [Crossref] [PubMed]
- Esch EW, Bahinski A, Huh D. Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov 2015;14:248-60. [Crossref] [PubMed]
- Ribas J, Sadeghi H, Manbachi A, et al. Cardiovascular Organ-on-a-Chip Platforms for Drug Discovery and Development. Appl In Vitro Toxicol 2016;2:82-96. [Crossref] [PubMed]
- Middelkamp HT, van der Meer AD, Hummel JM, et al. Organs-on-Chips in Drug Development: The Importance of Involving Stakeholders in Early Health Technology Assessment. Appl In Vitro Toxicol 2016;2:74-81. [Crossref]
- van de Burgwal LH, van Dorst P, Viëtor H, et al. Hybrid business models for ‘Organ-on-a-Chip’ technology: The best of both worlds. PharmaNutrition 2018;6:55-63. [Crossref]
- Zhang B, Radisic M. Organ-on-a-chip devices advance to market. Lab Chip 2017;17:2395-420. [Crossref] [PubMed]
- Polacheck WJ, Li R, Uzel SG, et al. Microfluidic platforms for mechanobiology. Lab Chip 2013;13:2252-67. [Crossref] [PubMed]
- Ribas J, Zhang YS, Pitrez PR, et al. Biomechanical Strain Exacerbates Inflammation on a Progeria-on-a-Chip Model. Small 2017;13: [Crossref] [PubMed]
- Huh D, Leslie DC, Matthews BD, et al. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med 2012;4:159ra47 [Crossref] [PubMed]
- Huh D, Matthews BD, Mammoto A, et al. Reconstituting organ-level lung functions on a chip. Science 2010;328:1662-8. [Crossref] [PubMed]
- Kim HJ, Huh D, Hamilton G, et al. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 2012;12:2165-74. [Crossref] [PubMed]
- Kim HJ, Li H, Collins JJ, et al. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc Natl Acad Sci U S A 2016;113:E7-15. [Crossref] [PubMed]
- Agarwal A, Goss JA, Cho A, et al. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip 2013;13:3599-608. [Crossref] [PubMed]
- Annabi N, Selimovic S, Acevedo Cox JP, et al. Hydrogel-coated microfluidic channels for cardiomyocyte culture. Lab Chip 2013;13:3569-77. [Crossref] [PubMed]
- Marsano A, Conficconi C, Lemme M, et al. Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissues. Lab Chip 2016;16:599-610. [Crossref] [PubMed]
- Wang G, McCain ML, Yang L, et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med 2014;20:616-23. [Crossref] [PubMed]
- Song JW, Munn LL. Fluid forces control endothelial sprouting. Proc Natl Acad Sci U S A 2011;108:15342-7. [Crossref] [PubMed]
- Yasotharan S, Pinto S, Sled JG, et al. Artery-on-a-chip platform for automated, multimodal assessment of cerebral blood vessel structure and function. Lab Chip 2015;15:2660-9. [Crossref] [PubMed]
- Zheng W, Huang R, Jiang B, et al. An Early-Stage Atherosclerosis Research Model Based on Microfluidics. Small 2016;12:2022-34. [Crossref] [PubMed]
- Zhou J, Niklason LE. Microfluidic artificial "vessels" for dynamic mechanical stimulation of mesenchymal stem cells. Integr Biol (Camb) 2012;4:1487-97. [Crossref] [PubMed]
- Schimek K, Busek M, Brincker S, et al. Integrating biological vasculature into a multi-organ-chip microsystem. Lab Chip 2013;13:3588-98. [Crossref] [PubMed]
- Junaid A, Mashaghi A, Hankemeier T, et al. An end-user perspective on Organ-on-a-Chip: Assays and usability aspects. Curr Opin Biomed Eng 2017;1:15-22. [Crossref]
- Zhang YS, Aleman J, Shin SR, et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc Natl Acad Sci U S A 2017;114:E2293-302. [Crossref] [PubMed]
- Gordon LB, Shappell H, Massaro J, et al. Association of Lonafarnib Treatment vs No Treatment With Mortality Rate in Patients With Hutchinson-Gilford Progeria Syndrome. JAMA 2018;319:1687-95. [Crossref] [PubMed]
- Cho H, Seo JH, Wong KH, et al. Three-Dimensional Blood-Brain Barrier Model for in vitro Studies of Neurovascular Pathology. Sci Rep 2015;5:15222. [Crossref] [PubMed]
- Brown JA, Pensabene V, Markov DA, et al. Recreating blood-brain barrier physiology and structure on chip: A novel neurovascular microfluidic bioreactor. Biomicrofluidics 2015;9:054124 [Crossref] [PubMed]
- Griep LM, Wolbers F, de Wagenaar B, et al. BBB on chip: microfluidic platform to mechanically and biochemically modulate blood-brain barrier function. Biomed Microdevices 2013;15:145-50. [Crossref] [PubMed]
- Lee JS, Romero R, Han YM, et al. Placenta-on-a-chip: a novel platform to study the biology of the human placenta. J Matern Fetal Neonatal Med 2016;29:1046-54. [Crossref] [PubMed]
- Sato M, Sasaki N, Ato M, et al. Microcirculation-on-a-Chip: A Microfluidic Platform for Assaying Blood- and Lymphatic-Vessel Permeability. PLoS One 2015;10:e0137301 [Crossref] [PubMed]
- Lee H, Cho DW. One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology. Lab Chip 2016;16:2618-25. [Crossref] [PubMed]
- Bhattacharjee N, Parra-Cabrera C, Kim YT, et al. Desktop-Stereolithography 3D-Printing of a Poly(dimethylsiloxane)-Based Material with Sylgard-184 Properties. Adv Mater 2018;30:e1800001 [Crossref] [PubMed]
- Miri AK, Nieto D, Iglesias L, et al. Microfluidics-Enabled Multimaterial Maskless Stereolithographic Bioprinting. Adv Mater 2018;e1800242 [Crossref] [PubMed]
- Verhulsel M, Vignes M, Descroix S, et al. A review of microfabrication and hydrogel engineering for micro-organs on chips. Biomaterials 2014;35:1816-32. [Crossref] [PubMed]
- Esch MB, King T, Shuler M. The role of body-on-a-chip devices in drug and toxicity studies. Annu Rev Biomed Eng 2011;13:55-72. [Crossref] [PubMed]
- Lee JB, Sung JH. Organ-on-a-chip technology and microfluidic whole-body models for pharmacokinetic drug toxicity screening. Biotechnol J 2013;8:1258-66. [Crossref] [PubMed]
- Sung JH, Srinivasan B, Esch MB, et al. Using physiologically-based pharmacokinetic-guided “body-on-a-chip” systems to predict mammalian response to drug and chemical exposure. Exp Biol Med (Maywood) 2014;239:1225-39. [Crossref] [PubMed]
- Wikswo JP, Curtis EL, Eagleton ZE, et al. Scaling and systems biology for integrating multiple organs-on-a-chip. Lab Chip 2013;13:3496-511. [Crossref] [PubMed]
- Wikswo JP, Block FE 3rd, Cliffel DE, et al. Engineering challenges for instrumenting and controlling integrated organ-on-chip systems. IEEE Trans Biomed Eng 2013;60:682-90. [Crossref] [PubMed]
- Sung JH, Shuler ML. A micro cell culture analog (microCCA) with 3-D hydrogel culture of multiple cell lines to assess metabolism-dependent cytotoxicity of anti-cancer drugs. Lab Chip 2009;9:1385-94. [Crossref] [PubMed]
- Zhang C, Zhao Z, Abdul Rahim NA, et al. Towards a human-on-chip: culturing multiple cell types on a chip with compartmentalized microenvironments. Lab Chip 2009;9:3185-92. [Crossref] [PubMed]
- Sung JH, Kam C, Shuler ML. A microfluidic device for a pharmacokinetic-pharmacodynamic (PK-PD) model on a chip. Lab Chip 2010;10:446-55. [Crossref] [PubMed]
- Zhang YS, Ribas J, Nadhman A, et al. A cost-effective fluorescence mini-microscope for biomedical applications. Lab Chip 2015;15:3661-9. [Crossref] [PubMed]
- Zhang YS, Busignani F, Ribas J, et al. Google Glass-Directed Monitoring and Control of Microfluidic Biosensors and Actuators. Sci Rep 2016;6:22237. [Crossref] [PubMed]
- Zhang YS, Khademhosseini A. Seeking the Right Context for Evaluating Nanomedicine: from Tissue Models in Petri Dishes to Microfluidic Organs-on-a-Chip. Nanomedicine 2015;10:685-8. [Crossref] [PubMed]
- Soscia D, Belle A, Fischer N, et al. Controlled placement of multiple CNS cell populations to create complex neuronal cultures. PLoS One 2017;12:e0188146 [Crossref] [PubMed]
Cite this article as: Ribas J, Pawlikowska J, Rouwkema J. Microphysiological systems: analysis of the current status, challenges and commercial future. Microphysiol Syst 2018;2:10.


